P A R T O N E
The Problem:
Nuclear Radiation and its Biological Effects
The Seed
The future of humankind is present today within the bodies of living
people, animals and plants -- the whole seedbearing biosphere. This
living biosystem which we take so much for granted has evolved
slowly into a relatively stable dynamic equilibrium, with predictable
interactions between plants and animals, between microscopic and
macroscopic life, between environmental pollutants and human
health. Changes in the environment disturb this balance in two
ways: first, by altering the carefully evolved seed by randomly
damaging it, and second, by altering the habitat, i.e. food, climate
or environment, to which the seed and/or organism has been
adapted, making life for future generations more difficult or even
impossible.
Although
examples of maladaptation in nature and resulting
species extinction abound, our focus here is on human seed, the
sperm and ovum, and the effect on it and on the human habitat
resulting from increasing ionising radiation in the environment.
The
increased use of radioactive materials, which is a direct
outgrowth of the current military and energy policies of the
developed world, provides an opportunity for gauging what priority
these countries give to the health and well-being of individual
citizens, and for gauging governments' understanding of the tension
between individual and national survival. The first indicator of
underlying national priorities is the precision or lack of precision
with which health effects are predicted, and the thoroughness with
which an audit is taken and the predictions checked against
reality. The audit findings should be reported to the person or people
affected, and their participation sought in formulating changes in
policy to remedy any unanticipated problems. The individual's
sense of self-preservation and personal benefit, in such an ideal system,
would give realistic feedback to governments on the acceptability of national
policy. The combined experiences of governing and governed would forge a
national consensus on future directions.
|
Glossary
|
The Fissioning Process and its Consequences
In order to understand nuclear technology and its impact on human health,
three atomic-level events must be understood: fissioning, activation and
ionisation. Fissioning, i.e. the splitting of the
uranium or plutonium atom, is responsible for producing radioactive fission
fragments and activation products. These in turn cause the ionisation of
normal atoms, leading to a chain of microscopic events we may eventually
observe as a cancer death or a deformed child.
Radioactive
fission products are produced in nuclear reactors. They are
variant forms of the ordinary chemicals which are the building blocks of all
material and living things. The radioactive forms of these chemicals were,
prior to 1943, present in only trace quantities in isolated places in the
environment as, for example, in South Africa where it appears that a small
nuclear fission reaction occurred spontaneously about 1700 million years ago.
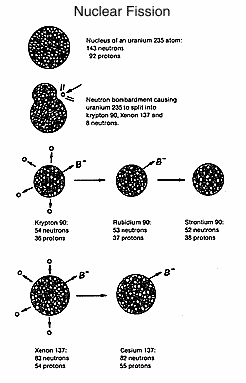
When
a uranium atom is split or fissioned, it does not always split
in the same place. The two pieces, called fragments, are chemicals of
lower atomic weight than uranium. Each fragment receives part of the
nucleus and part of the electrons of the original large uranium atom. The
uranium atoms, of course, cease to exist after they are split. Instead,
more than 80 different possible fission products are formed,
each having the chemical properties usually associated with their
structure, but having the added capability of releasing ionising
radiation. X-rays, alpha particles, beta particles, gamma rays (like
X-rays) or neutrons can be released by these `created' chemicals. All
these can cause `ionisation', i.e. by knocking an electron out of its
normal orbit around the nucleus of an atom they produce two `ions',
the negatively charged electron and the rest of the atom which now
has a net positive electrical charge.
The
atomic structure of fission fragments is unstable. The atom will
at some time release the destabilizing particle and return to a natural,
low-energy, more stable form. Every such release of energy is an
explosion on the microscopic level. With each fissioning, 2 or 3
neutrons are released which can strike a nearby U235 atom causing
more fissioning in what is usually called a chain reaction.
 The
violence of the chain reaction is such that it can also yield
what are called activation products, i.e. it can cause already existing
chemicals in air, water or other nearby materials to absorb energy,
change their structure slightly and become radioactive. As these
high-energy forms of natural materials eventually return to their normal
stable state, they can also release ionising radiation. About 300
different radioactive chemicals are created with each chain
reaction.[1]
It takes hundreds of
thousands of years for all the newly formed radioactive chemicals to
return to a stable state.
The
violence of the chain reaction is such that it can also yield
what are called activation products, i.e. it can cause already existing
chemicals in air, water or other nearby materials to absorb energy,
change their structure slightly and become radioactive. As these
high-energy forms of natural materials eventually return to their normal
stable state, they can also release ionising radiation. About 300
different radioactive chemicals are created with each chain
reaction.[1]
It takes hundreds of
thousands of years for all the newly formed radioactive chemicals to
return to a stable state.
In
a nuclear power plant the fissioning takes place inside the
zirconium or magnesium alloy cladding which encloses the fuel rods. Most
of the fission fragments are trapped within the rods. However,
the activation products can be formed in the surrounding air, water,
pipes and containment building. The nuclear plant itself becomes
unusable with time and must eventually be dismantled and isolated as
radioactive waste.
After
fissioning, the fuel rods are said to be `spent'. They contain the
greatest concentration of radioactivity of any material on the planet
earth -- many hundreds of thousands of times the concentration in
granite or even in uranium mill tailings (waste). The spent fuel rods
contain gamma radiation emitters (which are similar to X-ray
emitters) so they must not only be isolated from the
biosphere, but they must also be shielded with water and thick lead
walls. Direct human exposure to spent fuel rods means certain death.
In
reprocessing, spent fuel rods are broken open and the outer
cladding is dissolved in nitric acid. The plutonium is separated out for
use in nuclear weapons or for fuel in a breeder or mixed oxide nuclear
reactor. The remaining highly radioactive debris is stored as liquid in
large carbon or stainless steel drums, awaiting some kind of
solidification and burial in a permanent repository. Waste of lower
radioactivity is buried in dirt trenches or -- as in Windscale
(Sellafield) in England -- piped out to sea. The spent nuclear fuel rods
and liquid reprocessing waste are called `high level radioactive
waste'. It must be kept secure for hundreds of thousands of years --
essentially forever. Lower level waste may be equally long-lived, but
it is less concentrated.
In
above-ground nuclear weapon testing, there is no attempt to
contain any of the fission or activation products. Everything is
released into the air and on to the land. Some underground tests are
also designed to release most of the radioactive particles; these are
called crater shots or shots with unstemmed holes. Even when
below-ground shots are designed to be contained, they normally lose the
radioactive gases and some particulates. The radionuclides trapped in
the ground can also migrate downwards in the earth to water
reservoirs which provide irrigation and drinking water for human
purposes, although this process is slow. Radioactive debris piped out
to sea can be washed back on shore or can contaminate fish.
In
all nuclear reactions, some radioactive material -- namely the
chemically inert or so-called `noble' gases, other gases, radioactive
carbon, water, iodine, and small particulates of plutonium and other
transuranics (i.e. chemicals of higher atomic number than uranium) --
is immediately added to the air, water and land of the biosphere. In
the far-distant future, all the long-lived radioactive material, even that
now stored and trapped, will mix with the biosphere unless each
generation repackages it. Our planet earth is designed to recycle
everything.
The
radioactive chemicals which escape to the biosphere can
combine with one another or with stable chemicals to form molecules
which may be soluble or insoluble in water; which may be solids,
liquids or gases at ordinary temperature and pressure; which may be
able to enter into biochemical reactions or be biologically inert. The
radioactive materials may be external to the body and
still give off destructive penetrating radiation. They may also be taken
into the body with air, food and water or through an open wound,
becoming even more dangerous as they release their energy in close
proximity to living cells and delicate body organs. They may remain
near the place of entry into the body or travel in the bloodstream or
lymph fluid. They can be incorporated into the tissue or bone. They
may remain in the body for minutes or hours or a lifetime. In nuclear
medicine, for example, radioactive tracer chemicals are deliberately
chosen among those quickly excreted by the body. Most of the
radioactive particles decay into other radioactive `daughter' products
which may have very different physical, chemical and radiological
properties from the parent radioactive chemical. The average number
of such radioactive daughters of fission products produced before a
stable chemical form is reached, is four.
Besides
their ability to give off ionising radiation, many of the
radioactive particles are biologically toxic for other reasons. Radioactive
lead, a daughter product of the radon gas released by
uranium mining retains the ability to cause brain damage exercised by
non-radioactive lead. Plutonium is biologically and chemically
attracted to bone as is the naturally occurring radioactive chemical
radium. However, plutonium clumps on the surface of bone, delivering
a concentrated dose of alpha radiation to surrounding
cells, whereas radium diffuses homogeneously in bone and thus has
a lesser localized cell damage effect. This makes plutonium, because
of its concentration, much more biologically toxic than a comparable amount of radium. Some allowance for this physiological
difference has been made in setting plutonium standards, but there
is evidence that there is more than twenty times more damage
caused than was suspected at the time of standard
setting.[2]
The
cellular damage caused by internally deposited radioactive
particles becomes manifest as a health effect related to the particular
organ damaged. For example, radionuclides lodged in the bones can
damage bone marrow and cause bone cancers or leukaemia, while radionuclides
lodged in the lungs can cause respiratory diseases. Generalised
whole body exposure to radiation can be expressed as a stress related
to a person's hereditary medical weakness. Individual breakdown
usually occurs at our weakest point. In this way, man-made radiation
mimics natural radiation and causes the ageing or
breakdown process to be accelerated.
Radioactive Particles and Living Cells: Penetration Power
Radioactive fission products, whether they are biochemically inert or
biochemically active, can do biological damage when either outside
the body or within.
X-rays
and gamma rays are photons, i.e. high-energy light-waves. When
emitted by a source, for example, radium or cobalt, located
outside the body, they easily pass through the body, hence they are
usually called penetrating radiation. The familiar lead apron provided
for patients in some medical procedures stops X-rays from reaching
reproductive organs. A thick lead barrier or wall is used to protect the
X-ray technician. Because X-rays are penetrating, they can be used in
diagnostic medicine to image human bones or human organs made
opaque by a dye. These internal body parts are differentially
penetrable. Where bones absorb the energy, no X-rays hit the sensitive
X-ray film, giving a contrast to form the picture of the bones on the
radiation-sensitive X-ray plate. High-energy gamma rays, which easily
penetrate bone, would be unsuitable for such medical usage because
the film would be uniformly exposed. In photography jargon, the
picture would be a
`white out' with no contrasts. No radiation remains in the body after an
X-ray picture is taken. It is like light passing through a window. The
damage it may have caused on the way through, however, remains.
Some
radioactive substances give off beta particles, or electrons,
as they release energy and seek a stable atomic state. These are
small negatively charged particles which can penetrate skin but
cannot penetrate through the whole body as do X-rays and gamma
rays.
 Microscopic nuclear explosions of some radioactive chemicals
release high-energy alpha particles. An alpha particle, the nucleus of
a helium atom, is a positively charged particle. It is larger in size than
a beta particle, like a cannon-ball relative to a bullet, having
correspondingly less penetrating power but more impact. Alpha
particles can be stopped by human skin, but they may damage the
skin in the process. Both alpha and beta particles penetrate cell
membranes more easily than they penetrate skin. Hence ingesting,
inhaling or absorbing radioactive chemicals capable of emitting alpha
or beta particles and thereby placing them inside delicate body parts
such as the lungs, heart, brain or kidneys, always poses serious threats
to human health.[3]
Plutonium is an alpha emitter, and
no quantity inhaled has been found to be too small to induce lung
cancer in animals.
Microscopic nuclear explosions of some radioactive chemicals
release high-energy alpha particles. An alpha particle, the nucleus of
a helium atom, is a positively charged particle. It is larger in size than
a beta particle, like a cannon-ball relative to a bullet, having
correspondingly less penetrating power but more impact. Alpha
particles can be stopped by human skin, but they may damage the
skin in the process. Both alpha and beta particles penetrate cell
membranes more easily than they penetrate skin. Hence ingesting,
inhaling or absorbing radioactive chemicals capable of emitting alpha
or beta particles and thereby placing them inside delicate body parts
such as the lungs, heart, brain or kidneys, always poses serious threats
to human health.[3]
Plutonium is an alpha emitter, and
no quantity inhaled has been found to be too small to induce lung
cancer in animals.
The
skin, of course, can stop alpha or beta radiation inside the body
tissue from escaping outwards and damaging, for example, a baby one
is holding or another person sitting nearby. Also, it is impossible to
detect these particles with most whole body `counters' such as are
used in hospitals and nuclear installations. These counters can only
detect X-rays and gamma rays emitted from within the body.
Splitting
a uranium atom also releases neutrons, which act like
microscopically small bullets. Neutrons are about one-fourth the size
of alpha particles and have almost 2,000 times the mass of an
electron. If there are other fissionable atoms nearby (uranium 235 or
plutonium 239, for example) these neutron projectiles may strike
them, causing them to split and to release more neutrons. This is the
familiar chain reaction. It takes place spontaneously when fissionable
material is sufficiently concentrated, i.e. forms a critical mass. In a
typical atomic bomb the fissioning is very rapid. In a nuclear reactor,
water, gas or the control rods function to slow down or to absorb
neutrons and control the chain reaction.
Neutrons
escaping from the fission reaction can penetrate the
human body. They are among the most biologically destructive ot the
fission products. They have a short range, however, and in the
absence of fissionable material they will quickly be absorbed by
non-radioactive materials. Some of these latter become radioactive in the
process, as was noted earlier, and are called activation products.
Standard-setting Preliminaries
The complexity of setting health standards for exposure to the mixture
of radioactive chemicals and ionising particles released in fissioning
should be apparent. As a first move towards a reasonable subdivision of
the hazard itself, separate standard setting was done
for external radiation exposure, i.e. when the radioactive source was
outside the body, and internal radiation exposure, i.e. when the
radioactive source was inside the body.
Both
these categories can then be subdivided into exposures to
particular parts of the body or particular internal organs. The
biological effect of an X-ray of the pelvic area differs from the
biological effect of a dental X-ray, even if the radiation dose to the
skin is the same. Plutonium lodged in the lungs has a different
biological consequence from plutonium lodged in the reproductive
organs. One can also consider exposures to X-rays, gamma rays, alpha
or beta particles and neutrons separately, taking each as internal or
external to the body.
There
are further differences in health effects based on differences
between people receiving the radiation. Special consideration needs
to be given to those who, because of heredity or previous experience,
are more susceptible to further damage than the norm or average. Special
consideration should be given to an embryo or foetus, a young
child, the elderly or those chronically ill.
The
severity of health effects caused by internal exposures will
depend on the biological characteristic of the radioactive chemical
and the length of time it may be expected to reside in the
body. Radioactive cesium, for example, lodges in muscles and is probably
completely eliminated from the body in two years. Radioactive
strontium lodges in bone and remains there for a lifetime, constantly
irradiating the surrounding cells. The usual time required by the body
to rid itself of half the radioactive chemical is called the `biological
half-life' of that chemical.
Some
radiation health effects are observable in the persons
exposed; some effects are only seen in their children or grandchildren
because the damage was to sperm or ovum.
X-rays,
gamma rays and neutrons are able to inflict harm on
humans even when the radioactive chemical emitting them is outside
the body. Beta particles outside the body can cause serious burns and
other skin anomalies, including skin cancer. Ionising radiations
emitted from within the body by radioactive chemicals taken in by
inhalation, ingestion or absorption are even more damaging because
they are so close to delicate cell structures. The body is not able to
distinguish between radioactive and nonradioactive chemicals and
will as readily incorporate the one as the other into tissue, bone,
muscle or organs, identifying them as ordinary nutrients. The
radioactive chemicals remain in the body until biologically
eliminated in urine or faeces, or until they decay into other
chemical forms (which may or may not be radioactive). These
daughter products and their chemical and radiological properties may
be quite different from those of the parent radioactive chemical,
for example, radioactive carbon decays into nitrogen. Radiochemical
analysis of urine or faeces is the preferred test for
most types of internal contamination with alpha or beta particles.
Within the Living Cell
The chaotic state induced within a living cell when it is exposed to
ionising radiation has been graphically described by Dr Karl Z. Morgan
as a `madman loose in a library'.[4]
The result of cell exposure to these
microscopic explosions with the resultant sudden influx of random
energy and ionisation may be either cell death or cell alteration. The
change or alteration can be temporary or permanent. It can leave the
cell unable to reproduce (or replace) itself. Radiation damage can
cause the cell to produce a slightly different hormone or enzyme than
it was originally designed to produce, still leaving it able to reproduce
other cells capable of generating this same altered hormone or
enzyme. In time there may be millions of such altered cells. This latter
mechanism, called biological magnification, can cause some of the
chronic diseases and changes we usually associate with old age. One
very specific mutation which can occur within the cell is the
destruction of the cell's mechanism for resting which normally causes
it to cease reproductive activities after cell division. This inability to
rest results in a runaway proliferation of cells in one place, which, if
not destroyed, will form a tumour, either benign or malignant. The
abnormal proliferation of white blood cells is characteristic of
leukaemia; red blood cell proliferation results in what is called
polycythemia vera.
If
the radiation damage occurs in germ cells, the sperm or ovum, it
can cause defective offspring. The defective offspring will in turn
produce defective sperm or ova, and the genetic `mistake' will be
passed on to succeeding generations, reducing their quality of life
until the family line terminates in sterilisation and/or
death.[5]
A blighted or
abnormal embryonic growth can result in what is called a
hydatidiform mole instead of a baby.
Exposure
to radiation is also known to reduce fertility, i.e. women
become unable to conceive or give birth.
Radiation
can also damage an embryo or foetus while it is
developing within the mother's womb. This is called teratogenic
damage, or the child is said to have a congenital malformation rather
than genetic damage. This means the damage is not automatically
transmitted. For example, a deaf person, made so by a pre-birth
injury, may have children with normal hearing.
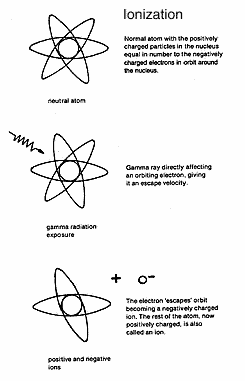 The
damage done within cells by random releases of the energy of
photons, alpha, beta or neutron particles can occur indirectly through
an effect called ionisation. As the energised photons or particles
speed through the cells, they give energy to the electrons of chemicals
already within the cells, enabling some electrons to break free from
the rest of the atom or molecule to which they are attached. On
the macro-level this would be comparable to an atomic explosion
of a magnitude great enough to drive the earth or another planet out of
its orbit around the sun. What was an electrically neutral atom or
molecule is split into two particles -- a larger positively charged atom
or molecule missing one of its electrons, and a small negatively
charged electron expelled from its orbit around the nucleus of the
atom. Both are called ions and the process is called ionisation.
The
damage done within cells by random releases of the energy of
photons, alpha, beta or neutron particles can occur indirectly through
an effect called ionisation. As the energised photons or particles
speed through the cells, they give energy to the electrons of chemicals
already within the cells, enabling some electrons to break free from
the rest of the atom or molecule to which they are attached. On
the macro-level this would be comparable to an atomic explosion
of a magnitude great enough to drive the earth or another planet out of
its orbit around the sun. What was an electrically neutral atom or
molecule is split into two particles -- a larger positively charged atom
or molecule missing one of its electrons, and a small negatively
charged electron expelled from its orbit around the nucleus of the
atom. Both are called ions and the process is called ionisation.
The
complex molecules making up living organisms are composed
of long strands of atoms forming proteins, carbohydrates and fats. They
are held together by chemical bonds involving shared electrons. If the
ionising radiation displaces one of the electrons in a chemical bond, it
can cause the chain of atoms to break apart, splitting the long
molecule into fragments, or changing its shape by elongation. This is
an `ungluing' of the complex chemical bonds so carefully structured to
support and perpetuate life. The gradual breakdown of these molecular
bonds destroys the templates used by the body to make DNA and RNA
(the information-carrying molecules in the cell) or causes abnormal
cell division. The gradual natural breakdown of DNA and RNA is
probably the cellular phenomenon associated with what we know as
`ageing'. It occurs gradually over the years with exposure to natural
background radiation from the radioactive substances which have
been a part of the earth for all known ages. There is evidence that
exposure to medical X-rays accelerates this breakdown
process.[6] There is ample
reason to think that fission products lodged within the
body will cause the same kind of acceleration of ageing. However,
unlike medical X-rays, these radioactive chemicals damage cells by
their chemical toxicity as well as their radiological properties.
The
gradual breakdown of human bio-regulatory integrity
through ionising and breakage of the DNA and RNA molecules
gradually makes a person less able to tolerate environmental changes,
less able to recover from diseases or illness, and generally less able to
cope physically with habitat variations.
When
the DNA of germ plasm is affected by radiation it can result
in chromosomal diseases, such as trisomy 21, more commonly known
as Down's Syndrome. Mentally retarded children, victims of Down's
Syndrome, have been reported in Kerala, India, an area of high natural
radioactivity.[7]
Recently, cases of Down's Syndrome have
been tentatively linked to women exposed to radioactive releases
from the large plutonium fire at Sellafield (Windscale) in
1957.[8]
While Down's Syndrome babies have long been associated with births to
older women (those with higher accumulated exposure to natural background
radiation),[9]
the Sellafield-related cases involve women with an average age of 25 years.
So
far we have considered the types of ionising radiation, the
location of the source outside or within the body, and the difference
between exposures to different parts of the body or to different people
of various ages and states of health. These will all be important
considerations underlying standard setting. Next, we need to be able
to measure radiation, i.e. to quantify exposure.
Measuring Radiation
One way to approach the measurement of radiation is to count the
number of nuclear transformations or explosions which occur in a
given unit of radioactive substance per second. This measure is
usually standardised to radium, the first radioactive substance to be
discovered and widely used. One gram of radium undergoes 3.7 x 10^10
nuclear transformations or disintegrations per second. The activity of 1
gram of radium is called 1 curie (Ci), named for Madame Marie
Curie, a Polish-born French chemist (1867-1934). Marie Curie
discovered the radioactivity of thorium, polonium and radium by
isolating radium from pitchblende. She and her daughter Irene were
among the earliest known radiation victims, both dying of aplastic
anaemia.
In
recent radiation protection guides, the curie is being replaced by
the becquerel, which indicates one atomic event per second. One
gram of radium would equal 1 curie of radium or 3.7 x 10^10 becquerels
of radium.
The
energy released in nuclear disintegrations has the ability to
do work, i.e. to move matter. In physics, the erg is a very small unit of
work done. Lifting 1 gram of radium 1 centimetre requires 980 ergs of
work. Any material exposed to the force from nuclear disintegrations
at a rate of 100 ergs/gm is said to absorb one rad, i.e. radiation
absorbed dose. There is no direct conversion from curies,
which is related to the number of atomic events, to the rad dose, which
is energy absorbed in tissue. The curie gives one an estimate of the
number of microscopic transformations or explosions per second and
the rad is an estimate of the energy release, absorbed by the
surrounding tissue. On the macro-level, the word `explosion' tells us
only of an event in time. A dynamite explosion or hydrogen bomb
explosion adds information about the energy released.
Sometimes
radioactivity is measured in counts per minute on a
Geiger counter. A nuclear transformation within an energy range
measured by the instrument and close enough to the instrument
causes a noise or `count'. Most Geiger counters cannot detect alpha
particle emitters like plutonium.
The
radioactivity of elements which experience nuclear
disintegrations is measured relative to radium. For example, it would
take more than 1 million grams of uranium to be equivalent in
radioactivity, i.e. to have the same number of nuclear events per
second as 1 gram of radium has per second. Both 1 million grams of
uranium and 1 gram of radium would be measured as 1 Ci. It has been
the custom in the past to limit human exposure to uranium more for its
toxic chemical properties (it is a heavy metal) than for its
radioactivity. This practice may have underestimated damage caused
by the biological storing of uranium in the liver.
When
uranium decays, it passes through about 12 radioactive forms,
called daughter products, before reaching a stable chemical form of
lead. One of the radioactive daughter products of uranium is
radium. Uranium released into drinking water or incorporated into food
and human tissue today will eventually plague the world as radium and its
other disintegration products: radon gas and the radioactive forms of
polonium, lead and bismuth. The environmental and biochemical
forces which may tend to reconcentrate these toxic materials in living
cells are not well known. Although uranium occurs naturally, it has
become much more available for entering into water, food, living cells
and tissue since the mining boom which began shortly after the
Second World War.
The
activity which takes place in the nucleus of the uranium or
radium atom is a `haphazard' event obeying the laws of random
probabilities. An atom is characterised by its atomic number, that
is, the positively charged particles in its nucleus, and by its atomic
mass, expressed in atomic mass units (similar to the concept of
weight), which includes both the number of protons (the atomic
number) and the number of neutrons in the nucleus. Carbon, the most
frequently occurring chemical in living material, is taken as having
exactly 12 atomic mass units and other atoms are measured in
relation to this. Carbon 14, which is radioactive, has two extra
neutrons in its nucleus.
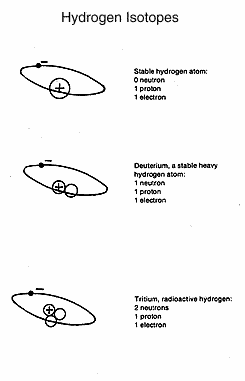 Hydrogen,
another example, has an atomic number of 1 and an
atomic mass of 1. Isotopes of hydrogen have the same atomic number
(that is, the same number of positively charged particles in the
nucleus and electrons in orbit around the nucleus) but a higher atomic
mass. Deuterium or hydrogen 2, an isotope of hydrogen, has an atomic
number of 1 and an atomic mass of 2. It is not radioactive. The
increased atomic mass is due to an added neutron in the
nucleus. Deuterium is in the `heavy water' used in the Canadian CANDU
nuclear reactor. Hydrogen 3, called tritium, is radioactive, with two
neutrons and a proton in the nucleus. It is produced in a nuclear
reaction.
Hydrogen,
another example, has an atomic number of 1 and an
atomic mass of 1. Isotopes of hydrogen have the same atomic number
(that is, the same number of positively charged particles in the
nucleus and electrons in orbit around the nucleus) but a higher atomic
mass. Deuterium or hydrogen 2, an isotope of hydrogen, has an atomic
number of 1 and an atomic mass of 2. It is not radioactive. The
increased atomic mass is due to an added neutron in the
nucleus. Deuterium is in the `heavy water' used in the Canadian CANDU
nuclear reactor. Hydrogen 3, called tritium, is radioactive, with two
neutrons and a proton in the nucleus. It is produced in a nuclear
reaction.
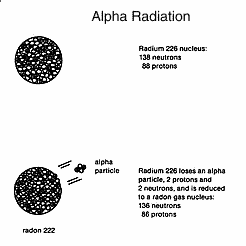
When
radium 226 decays, it loses a positively charged alpha
particle from its nucleus. An alpha particle has two protons (positive
electrical charges) and a mass of 4 atomic units. This means a
reduction in both radium's atomic number and atomic mass. Loss of
the alpha particle changes radium 226 (transmutes it) into another
element, radon 222. While radium 226 is a radioactive solid under
normal conditions, radon 222 is a radioactive gas. Loss of one or more
protons changes the chemical element into a different
chemical. Absorption or loss of a neutron gives an isotope of the
same chemical since chemical properties are determined by the number
of protons and electrons in an atom.
The
time required for half of any amount of radium 226 to transmute
to radon 222 by these small explosions which emit alpha particles
is 1,622 years. This is called the physical half-life of radium. Half
of the radium literally disappears in that length of time, but radon
gas is produced to replace it. Radon gas is radioactive and more
mobile in air and water (it dissolves) than the solid radium. The
half-life of radon is 3.82 days, after which half the gas will have
disintegrated, again releasing alpha particles and transmuting into
radioactive polonium 218, which is a solid. With a wind of 10 mph (or
kph), the radon gas could travel 1,000 miles (or kilometres) from the
point of origin before half of it would have decayed into its solid
daughter products and been deposited on soil, leafy vegetables,
tobacco, groundwater, human skin, lung tissue, etc. If the
material receiving the radioactive daughter product is living,
then it can carry the particles into its cells. Such contamination
cannot be washed off.
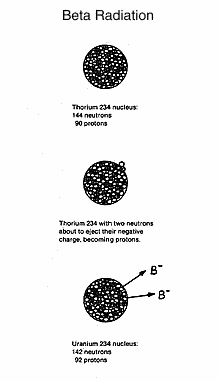 When
a negatively charged beta particle is released, there is a
transmutation in which a neutron in the nucleus of the atom splits into
a proton and an electron, the proton remaining in the nucleus and the
electron given off as a fast-moving microscopic bullet. Beta particles
are extremely small. The mass of an alpha particle is about 7,400
times that of a beta particle. Thorium 234 decays to uranium 234 (with
a short-lived radioactive intermediary) by losing beta particles. Uranium
and thorium are different elements, but have the same mass
(atomic weight) since a neutron and proton have about the same
mass. The thorium neutron becomes the uranium proton. The half-life
of thorium 234 is 24.1 days, while the half-life of uranium 234 is 2.50
x 10^5, or 250,000 years. As was pointed out earlier, uranium nuclear
events are not as frequent as those in radium, although they are
destructive when they occur.
When
a negatively charged beta particle is released, there is a
transmutation in which a neutron in the nucleus of the atom splits into
a proton and an electron, the proton remaining in the nucleus and the
electron given off as a fast-moving microscopic bullet. Beta particles
are extremely small. The mass of an alpha particle is about 7,400
times that of a beta particle. Thorium 234 decays to uranium 234 (with
a short-lived radioactive intermediary) by losing beta particles. Uranium
and thorium are different elements, but have the same mass
(atomic weight) since a neutron and proton have about the same
mass. The thorium neutron becomes the uranium proton. The half-life
of thorium 234 is 24.1 days, while the half-life of uranium 234 is 2.50
x 10^5, or 250,000 years. As was pointed out earlier, uranium nuclear
events are not as frequent as those in radium, although they are
destructive when they occur.
Given
12 grams of thorium 234, we would have 6 grams after 24.1
days, 3 grams after 48.2 days, 1.5 grams after 72.3 days, 0.75 grams
after 96.4 days, etc. At the same time, the stock of uranium 234 would
be increasing as the thorium decays into the new radioactive
chemical.
There
is no simple physical or chemical process such as
temperature change or chemical bonding which can prevent these
radioactive elements from decaying. Their nucleus is unstable and
because all elements seek a stable low-energy state, they must at
some time release particles in an effort to reach a resting state. The
decay takes place in the nucleus of the atom regardless of whether the
atom exists singly or is part of a molecule; is in the solid, liquid or
gaseous state; is within the body or outside, and so on. The decay
product after a radioactive disintegration may itself be radioactive, so
disintegration does not put an end to the biological problems
generated by these small explosions. This decay process must be
taken into account when estimating the biological effects of internal
exposure to radioactive material. Inhaled radon gas quickly becomes
radioactive lead, bismuth or polonium in the bloodstream.
One
should not confuse physical half-life with biological half-life,
i.e. the time required to eliminate half of the material from the body
through exhalation, urine or faeces. Cesium 137 and strontium 90 both
have physical half-lives of almost thirty years, but cesium 137 is
normally excreted from the body within two years while strontium 90
can be incorporated in bone for a lifetime.
One
more measure needs to be introduced before radiation protection
guides can be understood. Since the various kinds of radiation
exposures need to be evaluated for biological impact and not just for
the amount of energy absorbed by the tissue, the term rem, roentgen
equivalent man (or woman), was introduced. The rem dose is
the rad dose times a quality factor Q. For external radiation Q is
usually taken as 1, and rads and rems are used interchangeably. However,
to reflect the greater biological damage done by alpha
particles when inside the body, the rad dose may be multiplied by 20
to give the rem dose. This is another way of saying that the alpha
particle does damage of an order of magnitude (20 times) greater
when lodged within a tissue, bone or organ. For example, alpha
particles giving a 2 rem (or rad) dose to skin would give a 40 rem
dose to sensitive lung tissue when inhaled.
Theoretically,
the rem dose measures equivalent biological effect,
so that damage from X-rays, for example, would be the same as damage
from alpha particles, when the dose in rem was the same. Unfortunately,
living systems are too complex for such an approach to
provide anything more than a good guess.
Sometimes
references are made to a `fifty-year effective dose
equivalent'. This is the full dose that would be received from an
internal radionuclide if the dose were given at one time instead of
being spread over two to fifty years.
Linear Energy Transfer (LET)
Measurement of the number of ionisations which radiation causes per
unit distance as it traverses the living cell or tissue is called the
linear energy transfer of the radiation. The concept involves lateral
damage along the path, in contrast to path length or penetration
capability. Medical X-rays and most natural background radiation are
low LET radiation, while alpha particles have high LET. On the
average, fission fragments have high LET.
The
density of ionisation causes special problems in sperm and ova
because the damage (protein breakage) is concentrated within a few
cells. The two-year sterility of Japanese fishermen exposed to fallout
from the 1954 hydrogen bomb test is probably an example of this
effect. Sperm and the cells which produce sperm were damaged
beyond their capability of prompt repair.
As
a young girl in St George, Utah, USA, Elizabeth Catalan used
to stand outdoors and watch the mushroom clouds raised by the
Nevada nuclear tests float overhead. She has never been able to have
children. She, like some other women in St George, is unable to carry
a foetus to birth. Elizabeth's father, president of a local college, died
prematurely of leukaemia. He used to go horse-riding with three
friends and was frequently outside when the grey clouds laden with
radioactive chemicals went over. Three of the four men are now dead
from cancer.
Elizabeth's
sister died in her late twenties of a thyroid disease which
may have been caused by the radioactive iodine released in the
atomic blasts. Elizabeth and her mother attribute many of their
abnormal health problems, and those of family and friends, to the
atomic fallout. No government studies have been undertaken to
confirm or deny these claims. However, the situation was so widely
recognised as abnormal by the local population that the Governor of
Utah has filed a court claim against the US Federal Government for
wrongful deaths of the people of Utah. About a thousand individual
damage claims have entered the courts in the USA, and as part of the
trial preparations Dr Carl Johnson undertook a detailed study of the
Mormon population of Utah exposed to the fallout. It is reasonable to
conclude that the health problems reported by the people of Utah are
typical of what could be expected on the basis of theoretical
radiobiology.[10]
On
10 May 1984, US District Court Judge Bruce S. Jenkins ruled on
the first twenty-four claims of US government negligence in its
conduct of nuclear testing. He has awarded $2.6 million in damages to
ten claimants. This landmark, 489-page, carefully worded decision is
expected to be appealed against by the US Federal Government.
In
order to have a quantitative sense of the frequency of the
different cell effects caused by radiation exposure, imagine a colony
of 1,000 living cells exposed to a 1 rad X-ray (about the dose for one
X-ray spinal examination). There would be two or three cell deaths,
two or three mutations or irreparable changes in cell DNA and about
100,000 ionisations in the whole colony of cells -- ranging from 11 to
460 ionisations per cell.[11]
While cells can repair some damage, no one claims that there is
perfect repair even after only one such X-ray.
A
comparable 1 rad exposure to neutrons which have higher linear
energy transfer (LET) would be expected to cause more cell deaths
and more mutations. The ionisations caused would range
from 145 to 1,100 per cell.
Alpha
particles which occur naturally would cause roughly 10
times as many cell deaths and mutations, and 3,700 to 4,500
ionisations per cell. Alpha particles have high linear energy transfer.
The
average number of cell deaths and mutations caused by fresh
fission particles (i.e. those present soon after detonation of a nuclear
bomb) would be even greater, with the ionisations as frequent as
130,000 per cell.[12]
In nuclear reactors, most of these
extremely high-energy early fission fragments are enclosed within the
fuel rod. In a nuclear bomb blast, they are all released but they
decay very quickly and do not persist long in the environment.
If
instead of thinking of a colony of living cells, we think of a
person exposed to 1 rad (again about the skin dose from one spinal
X-ray) of 1 MeV (million electron volts) energy, this corresponds to 2.2
billion (US) photons per cm^2 acting on the body. In the words of Karl
Morgan, `It is inconceivable that all the billions of irradiated and
damaged cells would be completely
repaired.'[13]
This unrepaired damage accumulates,
eventually causing a reduction in the level of health that is normal
for a particular age.
Stated
very simply, ionising radiation seriously disrupts the chemistry
of the cell. It can also kill or permanently change the cell. Every
exposure to ionising radiation has this effect, and it is not
possible for the body to perfectly repair all of the damage. Whether or
not the residual unrepaired damage is of concern to the individual
exposed is a personal value judgment. It is not at all clear that
ordinary people find the damage `acceptable" unless it initiates a fatal
cancer, and yet this is the basis on which radiological safety
standards are set in all nations of the world.
R.
M. Sievert, the famous radiologist, who had supervised radiation
therapy since 1926 at the Karolinska Institute in Stockholm, pointed
out at an international meeting in 1950 that `there is no known
tolerance level for radiation'.[14]
A tolerance level is a level below
which there is no damage (sometimes called a threshold). A safety
level is ordinarily a fraction (one-tenth) of the tolerance
level.[14]
Cell Damage Expressed as a Health Problem
An example to show the connection between cell damage and
observable illness in the person exposed might help in understanding
the problems posed by radionuclide (radioactive chemical)
uptake, i.e. their ingestion, inhalation or absorption with food, air and
water, into human bodies, with subsequent cell damage. The thyroid
gland contains cells which produce thyroid hormone, which when released
into the bloodstream causes the body functions such as breathing,
digesting and reacting to stress to proceed at a certain rate. If
the thyroid is `overactive', one might notice in the person increased
pulse rate, nervousness, excitability, loss of body weight and, in
females, more frequent menstruation. Such a person is often called
`hyperactive' (hyper-thyroidism). A normal amount of thyroid hormone
in the blood produces a normally active individual. An `underactive' or
`hypoactive' thyroid can result in sluggishness, listlessness, weight
gain and irregular and/or infrequent menstrual flow in women
(hypothyroidism).
If
radioactive iodine (I 131 or I 129) is ingested with food it will
enter the blood and tend to accumulate in the thyroid. Radioactive
iodine emits high-energy gamma radiation which can destroy thyroid
cells, thus reducing total thyroid hormone production in the individual
so affected.
A
small amount of radioactive iodine would probably kill only a few
cells and have little or no noticeable effect on health. However, if
many cells are destroyed or altered, the hormone level would
noticeably drop or the hormone itself would be slightly changed. The
individual would become lethargic and gain weight. If properly
diagnosed and severe enough to require medical intervention, this
hypoactive thyroid condition can be controlled with artificially
ingested thyroid hormone. A mild exposure experienced by a large
population could cause a decrease in average thyroid hormone levels
and an increase in average body weight, such as is occurring now in
the North American population. The USA has been polluted with
nuclear industries since 1943 and with radioactive iodine from weapon
testing since 1951. Radioactive iodine is routinely released in small
quantities by nuclear power plants and in large quantities by nuclear
reprocessing plants. It is not part of the natural human environment. The
connection between this pollution and the overweight problem
has, unfortunately, never been seriously researched. There is no
evidence to confirm or deny the hypothesis, but weight increase is a
well-known biological response to radioactive iodine. The hypothesis is
certainly plausible under the circumstances.
It
is possible for thyroid cells to be altered but not killed by the
radiation. The cellular growth mechanism may be damaged, allowing
a runaway proliferation of cells. This results in a thyroid tumour,
either cancerous (malignant), or non-cancerous (benign). Other
possible radiation damage includes changes in the chemical
composition of the individual's thyroid hormone, altering its action in
the body and causing clinically observable symptoms not easily
diagnosed or corrected.
There
is an extremely remote possibility that these changes will be
desirable, but the overall experience of randomly damaging a complex
organism like the human body is that it is destructive of health.
An
atomic veteran who participated in the nuclear tests which were
conducted by the USA in the Bikini atoll in the late 1940s reported
that he gained 75 lbs in the four years following his participation. The
doctor diagnosed his problem as hypothyroidism. He also suffered from
high blood pressure, chronic asthma and frequent bouts of bronchitis
and pneumonia. He has had six tumours diagnosed since 1949, when
he returned home from military service. Four have been surgically
removed.
Damage
to the thyroid of a developing foetus can cause mental
retardation and other severe developmental
anomalies.[15]
Other
radionuclides will lodge in other parts of the body. If the
trachea, bronchus or lung are exposed, the damage eventually causes
speech or respiratory problems. If radioactive particles lodge in the
stomach or digestive tract, the heart, liver, pancreas or other internal
organs or tissues, the health problems will be correspondingly different
and characteristic of the organ damaged. Radionuclides which lodge
in the bone marrow can cause leukaemia, depression of the immune
system (i.e. the body's ability to combat infectious diseases) or blood
diseases of various kinds.
If
the radiation dose is high, there is extensive cell damage and
health effects are seen immediately. Penetrating radiation doses at
1,000 rad or more cause `frying of the brain' with immediate brain
death and paralysis of the central nervous system. This is why no one
dared to enter the crippled Three Mile Island nuclear reactor building
during the 1979 accident. An average of 30,000 roentgens (or rads)
per hour were being reported by instruments within the containment
building. This would convert to a 1,000 rad exposure for two minutes
spent inside the building. Such a dose to the whole body is invariably
fatal.
The
radiation dose at which half the exposed group of people would
be expected to die, i.e. the 50 percent lethal dose, is 250 rad. The
estimate is somewhat higher if only young men in excellent health
(e.g. soldiers) are exposed. Between 250 and 1,000 rad,
death is usually due to gross damage to the stomach and gut. Below
250 rad death is principally due to gross damage to the bone marrow
and blood vessels. A dose of about 200 rad to a foetus in the womb is
almost invariably fatal.
Penetrating
radiation in doses above 100 rad inflicts severe skin
burns. Lower doses produce burns in some people. Vomiting and
diarrhoea are caused by doses above about 50 rad. There are some
individuals who are more sensitive to radiation, however, showing
typical vomiting and diarrhoea radiation sickness patterns with doses
as low as 5 rad. An individual may react differently at different times
of life or under different circumstances. Below 30 rad, for most
individuals, the effects from external penetrating radiation are not
immediately felt. The mechanism of cell damage is similar to that
described for minute quantities of radioactive chemicals which lodge
within the body itself, and our bodies are incapable of `feeling'
damage to or death of cells. Only when enough cells are damaged to
interfere with the function of an organ or a body system does the
individual become conscious of the problem.
By
sharpening our perceptions more subtle radiation effects can
often become observable where once they went unnoticed. For
example, a series of X-rays received by a young child may cause
temporary depression of the white blood cells, and ten days to two
weeks after the exposure the child will get influenza or some other
infectious disease. Ordinarily the parent views the two events as
unconnected.
Sometimes
one can observe a mutation in a person who has
experienced loss of hair after radiation therapy to kill tumour
cells: hair that was formerly very straight can be curly when
it grows again.
A
plant whose flowers are normally white with red tips but which
begins to form uniformly red flowers has mutated. Such an event has
been observed by persons living in the vicinity of Sellafield in the
United Kingdom.
The
use of radiation therapy to destroy malignant cells also has
observable results. It is rather like surgery in that it is deliberately
used to kill the unwanted tumour cells.
Probable Health Effects resulting
from Exposure to Ionising Radiation
|
Dose in rems
(whole body) |
Health effects
Immediate |
Delayed |
|
|
||
| 1,000 or more |
Immediate death.
`Frying of the brain'. |
None |
| 600-1,000 | Weakness, nausea, vomiting and diarrhoea followed by apparent improvement. After several days: fever, diarrhoea, blood discharge from the bowels, haemorrhage of the larynx, trachea, bronchi or lungs, vomiting of blood and blood in the urine. | Death in about 10 days. Autopsy shows destruction of hematopoietic tissues, including bone marrow, lymph nodes and spleen; swelling and degeneration of epithelial cells of the intestines, genital organs and endocrine glands. |
| 250-600 | Nausea, vomiting, diarrhoea, epilation (loss of hair), weakness, malaise, vomiting of blood, bloody discharge from the bowels or kidneys, nose bleeding, bleeding from gums and genitals, subcutaneous bleeding, fever, inflammation of the pharynx and stomach, and menstrual abnormalities. Marked destruction of bone marrow, lymph nodes and spleen causes decrease in blood cells especially granulocytes and thrombocytes. |
Radiation-induced
atrophy of the
endocrine glands
including the
pituitary, thyroid
and adrenal glands.
From the third to fifth week after exposure, death is closely correlated with degree of leukocytopenia. More than 50% die in this time period. Survivors experience keloids, ophthalmological disorders, blood dyscrasis, malignant tumours, and psychoneurological disturbances. |
| 150-250 | Nausea and vomiting on the first day. Diarrhoea and probable skin burns. Apparent improvement for about two weeks thereafter. Foetal or embryonic death if pregnant. |
Symptoms of malaise
as indicated above.
Persons in poor
health prior to
exposure, or those
who develop a
serious infection,
may not survive.
The healthy adult recovers to somewhat normal health in about three months. He or she may have permanent health damage, may develop cancer or benign tumours, and will probably have a shortened lifespan. Genetic and teratogenic effects. |
| 50-150 | Acute radiation sickness and burns are less severe than at the higher exposure dose. Spontaneous abortion or stillbirth. | Tissue damage effects are less severe. Reduction in lymphocytes and neutrophils leaves the individual temporarily very vulnerable to infection. There may be genetic damage to offspring, benign or malignant tumours, premature ageing and shortened lifespan. Genetic and teratogenic effects. |
| 10-50 | Most persons experience little or no immediate reaction. Sensitive individuals may experience radiation sickness. | Transient effects in lymphocytes and neutrophils. Premature ageing, genetic effects and some risk of tumours. |
| 0-10 | None | Premature ageing, mild mutations in offspring, some risk of excess tumours. Genetic and teratogenic effects. |
Radiation and Heredity
In 1943, Hermann Müller received a Nobel Prize for his work on the
genetic effects of radiation and was a dominant figure in developing
early radiation exposure recommendations made by the International
Commission on Radiological Protection
(ICRP).[16]
He showed through his work with Drosophila, a fruit fly, that ionising
radiation affects not only the biological organism which is exposed
but also the seed within the body from which the future generations
are formed.
In
1964 Hermann Müller published a paper, `Radiation and
Heredity', spelling out clearly the implications of his research for
genetic effects (damage to offspring) of ionising radiation on the human
species.[17]
The paper, though accepted in medical/biological circles, appears not to
have affected policy makers in the political or
military circles who normally undertake their own critiques of
published research. Müller predicted the gradual reduction of the
survival ability of the human species as several generations were
damaged through exposure to ionising radiation. This problem of
genetic damage continues to be mentioned in official radiation-health
documents under the heading `mild
mutations'[18]
but these mutations are not `counted' as health effects when standards are
set or predictions of health effects of exposure to radiation are made.
There is a difficulty in distinguishing mutations caused artificially by
radiation from nuclear activities from those which occur naturally from
earth or cosmic radiation. A mild mutation may express itself in
humans as an allergy, asthma, juvenile diabetes, hypertension,
arthritis, high blood cholesterol level, slight muscular or bone defects,
or other genetic `mistakes'. These defects in genetic make-up leave
the individual slightly less able to cope with ordinary stresses and
hazards in the environment. Increasing the number of such genetic
`mistakes' in a family line, each passed on to the next generation,
while at the same time increasing the stresses and hazards in the
environment, leads to termination of the family line through eventual
infertility and/or death prior to reproductive age. On a large scale, such
a process leads to selective genocide of families or species
suicide.[19]
It
soon became obvious that the usual method determining a
tolerance level for human exposure to toxic substances was
inappropriate for ionising radiation. The health effects were similar to
normally occurring health problems and were quite varied, ranging
from mild to severe in a number of different human organ systems,
and their appearance could be delayed for years or even generations.
Permissible Levels of Exposure
The US National Council on Radiation Protection and Measurement gave expression to the theoretical resolution of this human dilemma by articulating the implicit reasoning behind subsequent radiation protection standards development:[20]
-
A value judgment which reflects, as it were, a measure of
psychological acceptability to an individual of bearing slightly
more than a normal share of radiation-induced defective
genes.
-
A value judgment representing society's acceptance of
incremental damage to the population gene pool, when
weighted by the total of occupationally exposed persons, or
rather those of reproductive capacity as involved in Genetically
Significant Dose calculation.
- A value judgment derived from past experience of the somatic effects of occupational exposure, supplemented by such biomedical and biological experimentation and theory as has relevance.
This is now an internationally accepted approach to setting standards for toxic substances when no safe level of the substance exists.
In
short, this elaborate philosophy recognises the fact that there is
no safe level of exposure to ionising radiation, and the search for
quantifying such a safe level is in vain. A permissible level, based on
a series of value judgments, must then be set. This is essentially a
trade-off of health for some `benefit' -- the worker receives a livelihood,
society receives the military `protection' and electrical power is
generated. Efforts to implement these permissible standards would
then logically include convincing the individual and society that the
`permissible' health effects are acceptable. This has come to mean
that the most undesirable health effects will be infrequent and in line
with health effects caused by other socially acceptable
industries. Frequently, however, the worker and/or public is given
the impression that these `worst' health effects are the only individual
health effects. A second implication of the
standards-based-on-value-judgments approach is that unwanted
scientific research resulting in public scrutiny of these value
judgments must be avoided.
The
genetic effect considered by standard setters as most
unacceptable is serious transmittable genetic disease in live-born
offspring. These severely damaged children are usually a source of
suffering for the family and an expense for society which must provide
special institutions for the mentally and physically disabled. Severely
handicapped people rarely have offspring; many die, are sterile or are
institutionalised before they are able to bear children. Workers and
the public are told that the probability of having such severely
damaged offspring after radiation exposure within permissible levels is
slight. By omission, a mildly damaged child or a miscarriage is
implied to be `acceptable'.
|
From a column in the Yomiuri Shinbun (19 January 1965; evening edition)
A nineteen-year-old girl in Hiroshima committed suicide after
leaving a note: `I caused you too much trouble, so I will die as
I planned before.' She had been exposed to the atomic bomb
while yet in her mother's womb nineteen years ago. Her
mother died three years after the bombing. The daughter
suffered from radiation illness; her liver and eyes were affected
from infancy. Moreover, her father left home after the mother
died. At present there remain a grandmother, age seventy-five; an
elder sister, age twenty-two; and a younger sister,
age sixteen. The four women had eked out a living with their
own hands. The three sisters were all forced to go to work
when they completed junior high school. This girl had no time
to get adequate treatment, although she had an A-bomb
victim's health book.
|
Standard
setters judge that the most severe damage done directly to
the person exposed is a fatal radiation-induced cancer, and again, this
is a rare occurrence when exposure is within permissible levels. All
other direct damage is by omission considered `acceptable'.
In
its 1959 report recommending occupational standards for internal
radiation doses (i.e. radioactive chemicals which are permitted to
enter the body through air, water, food or an open wound), the
International Commission on Radiological Protection (ICRP) formed
the following definition:
A permissible genetic dose [to sperm and ovum], is that dose [of ionising radiation], which if it were received yearly by each person from conception to the average age of childbearing [taken as 30 years], would result in an acceptable burden to the whole population.[16] [Emphasis added.]
This
might be paraphrased to say that the general public
(governments) may be willing to accept the number of blind, deaf,
congenitally deformed, mentally retarded and severely diseased
children resulting from the permissible exposure level. Defined this
way, the problem becomes primarily an economic one, since society
needs to estimate the cost of providing services for the severely
disabled. Once reduced to an economic problem, some nations may
choose to promote early detection of foetal damage during pregnancy
and induced abortion when serious handicap is suspected. When a
foetus is aborted prior to sixteen weeks' gestation the event may not
need to be reported and included in vital statistics. It becomes a
non-happening, and the nation appears to be in `good health', having
reduced the number of defective births.
Mild
mutations, such as asthma and allergies, are ordinarily not
even counted as a `cost' of pollution. The economic burdens, `health
costs', fall more on the individual and family than on the
government. Their pain and grief do not appear in the risk/benefit
equation. Parents and children are unaware of the `acceptable
burden' philosophy.
The
prediction of the magnitude of the burden of severe genetic
ills on an exposed population is essential to this philosophy. However,
the data accumulated at Hiroshima and Nagasaki did not give clear
answers. Either through ineptitude or loss of survivors of the bombing,
who died before their story was told, the researchers failed to find
any severe genetic ills clearly attributable to the parental exposure to
radiation at low doses.[21]
Probably the more fragile individuals in the population died from the blast,
fire and trauma of the bombs, the women not surviving long enough to become
pregnant.[22]
Governments
could not use the research on genetic damage in
children of medical radiologists,[23]
although this damage was measurable, because, in the early days,
radiation exposure to physicians was not measured. No quantitative
dose/response estimates could be derived.
Animal
studies of radiation-related genetic damage abounded, and
the recommending body, ICRP, used (and still uses) mouse studies as
a basis of its official predictions of the severe genetic effects of
ionising radiation in humans.
As
late as 1980, a US National Academy of Science publication
from its committee on the Biological Effects of Ionising
Radiation[24]
stated:
New data on induced, transmissible genetic damage expressed in first generation progeny of irradiated male mice now allow direct estimation of first generation consequences of gene mutations on humans . . . As with BEIR I, a major obstacle continues to be the almost complete absence of information on radiation-induced genetic effects in humans. Hence, we still rely almost exclusively on experimental data, to the extent possible from studies involving mammalian species [i.e. mice].
These
mouse studies are used as the basis of prediction, and
permissible doses are set so that the expected number of severe
transmittable genetic effects in children of those exposed could be
presumed to be an acceptable burden for governments choosing a
nuclear strategy.
The
introductory section of ICRP Publication 2, 1959, states:
The permissible dose for an individual is that dose, accumulated over a long period of time or resulting from a single exposure, which, in the light of present knowledge carries a negligible probability of severe somatic [damage to the individual] or genetic [damage to the offspring] injuries, furthermore, it is such a dose that any effects that ensue more frequently are limited to those of a minor nature that would not be considered unacceptable by the exposed individual and by competent medical authorities. Section 30.[16] [Emphasis added.]
Mild mutations are notably happenings of a minor nature, normally neither reported nor monitored in the population. They are likely to be statistically hidden by normal biological variations and unconnected in the mind of the individual or his/her physician with the exposure. The publication continues:
The permissible doses can therefore be expected to produce effects [illnesses] that could be detectable only by statistical methods applied to large groups. Section 31.[16] [Emphasis added.]
In
spite of this clarity, no such statistical audit of all health effects
including chronic diseases in exposed people and mild mutations in
their offspring has ever been done. More than 25 years have expired
since this document was published and the world is more than 35
years into the nuclear age.
As
late as 1965, ICRP Publication
9[25]
stated:
The commission believes that this level [5 rems radiation exposure per 30 years for the general public] provides reasonable latitude for the expansion of atomic energy programs in the foreseeable future. It should be emphasised that the limit may not in fact represent a proper balance between possible harm and probable benefit because of the uncertainty in assessing the risks and benefits that would justify the exposure. [Emphasis added.]
The
committee protected itself against accusations of wrongdoing
but failed to protect the public from its possible error. It defines its
role as recommending, with the responsibility of action to protect
worker and public health resting with individual national
governments. Governments in turn tend to rely on ICRP
recommendations as the best thought of internationally respected experts.
In
spite of this uncertainty about responsibility and safety levels for
exposure of the public, 5 rem per year, rather than per 30 years, was
permitted for workers in the nuclear industry. The 5 rem per 30 years
was set as the average dose to a population, with a maximum of 0.5
rem per year (15 rem per 30 years) for any individual member of the
public.
For
twenty years, between 1945 and 1965, health research on the
effects of ionising radiation exposure has focused on estimating (not
measuring) the number of excess radiation-induced fatal cancers and
excess severe genetic diseases to be expected in a population (i.e. a
whole country) given the average estimated exposure to radiation for
the country. Disputes among scientists usually have to do with the
magnitude of these numbers. Omitted from this research are other
radiation-related human tragedies such as earlier occurrence of
cancers which should have been deferred to old age or even might not
have occurred at all because the individual would have died naturally
before the tumour became life-threatening. These are not excess
cancers, they are accelerated cancers. This
approach also omits other physiological disorders such as
malfunctioning thyroid glands, cardio-vascular diseases, rashes and
allergies, inability to fight off contagious diseases, chronic respiratory
diseases and mildly damaged or diseased offspring. The implications
of such `mild' health effects on species survival seem to have either
escaped the planners of military and energy technology, or to have
been deliberately not articulated. Other obvious limitations of this
national averaging approach include the failure to deal with global
distribution of air and water with the result that deaths and the
cumulative damage to future generations are not limited to one
country.
The
usual procedure for setting the standard for a toxic substance or
environmental hazard is to decide the relevant medical symptoms of
toxicity and determine a dose level below which these symptoms do
not occur in a normal healthy adult. This cut-off point is sometimes
called the tolerance level and it represents a sort of guide to the
human ability to compensate for the presence of the toxic substance
and maintain normal health. The tolerance level for a substance, if
one can be determined, is then divided by a factor (usually 10) to
give a safe level. This allows for human variability with respect to the
tolerance level and also for biological damage which may occur
below the level at which there are visible signs of toxicity,
i.e. sub-clinical toxicity.
Human
experience with ionising radiation had been recorded for
more than fifty years prior to the nuclear age, the early history of
handling radioactive material having been fraught with tragedy. The
discoverer of the X-ray, W. K. Roentgen, died of bone cancer in 1923,
and the two pioneers in its medical use, Madame Marie Curie and her
daughter, Irene, both died of aplastic anaemia at ages 67 and 59
respectively. At that time, bone marrow studies were rarely done, and
it was difficult, using blood alone, to distinguish aplastic anaemia
from leukaemia. Both diseases are known to be radiation-related. Stories
of early radiologists who had to have fingers or arms
amputated abound. There were major epidemics among radiation
workers, such as that among the women who painted the radium dials
of watches to make them glow in the dark. Finally, there were the
horrifying nuclear blasts in Hiroshima and Nagasaki.
The
painful period of growth in understanding the harmful effects of
ionising radiation on the human body was marked by periodic
lowering of the level of radiation exposures permitted to workers in
radiation-related occupations. For example, permissible occupational
exposure to ionising radiation in the United States was set at 52
roentgen (X-ray) per year in
1925,[26]
36 roentgen per year in
1934,[27]
15 rem per year in
1949[28]
and 5 to 12 rem per year from 1959 (depending on
average per year over age 18) to the
present.[29]
Recently there has been an effort to increase permissible doses of
ionising radiation to certain organs such as thyroid and bone
marrow[30] in
spite of research showing the radiosensitivity of these
tissues. This newer trend probably reflects economic rather than
physiological pressures, especially given the lack of an acceptable
audit of physiological cost.
Radiation Protection Standards
In 1952 the International Commission on Radiological Protection
(ICRP) issued its recommendations for limiting human exposure to
external sources of radiation. The newly formed organisation accepted
the standard agreed upon by nuclear physicists from the USA, Canada
and the UK after the Second World
War.[31]
In 1959 it issued its
recommendations for limiting human exposure to internal sources of
radiation. The early ICRP dose limits per year were: 5 rem to the
whole body, gonads or active bone marrow; 30 rem to bone, skin or
thyroid; 75 rem to hands, arms, feet or legs; and 15 rem to all other
body parts. These standards applied only to `man-made' sources, other
than medical exposures for diagnostic or therapeutic purposes of
benefit to the patient exposed.
ICRP
Publication 2, in 1959, recommended no more than 5 rem per
year external or internal exposure to the whole body due to inhalation,
ingestion or absorption of radioactive chemicals into the body. Sometimes
this was misinterpreted and workers were permitted to
receive up to 5 rem internal and 5 rem external radiation exposure
during one year. Another clause allowing averaging doses over years
beyond age 18, gave excuse for still higher doses.
In
terms of the amount of whole body dose received in a chest
X-ray (about 0.03 rem at the present time), this recommendation for
workers allowed the equivalent of 400 chest X-rays in some years
with a 170 (present-day) chest X-ray average (external and internal)
dose a year. Prior to 1970 some X-ray machines used in mass chest
X-ray programmes gave as high as 3 rem per chest X-ray.
When
one looks at dose to bone marrow, the permissible levels
are even more troubling. By 1970 the average bone marrow dose for a
chest X-ray was 0.001 to 0.006 rem averaging about 0.005 rem. In
terms of dose to bone marrow, the ICRP radiation recommendation for
workers permits up to the equivalent bone marrow dose of 1,000 chest
X-rays per year.
ICRP
recommended that members of the general public should
receive no more than one-tenth of the occupational exposure or 0.5
rem per year, the equivalent bone marrow dose of about 100 present-day
chest X-rays per year. The bone marrow dose is important for
estimating the likelihood of causing bone cancer, leukaemia, aplastic
anaemia or other blood disorders. Medical X-rays are less penetrating
of bone than of soft tissue, making them valuable for `picturing' the
bones. For this reason comparisons between radiation exposures of
nuclear workers and medical X-ray exposures are more appropriately
based on the bone marrow dose of each than on dose to soft tissue.
These
radiation exposure recommendations stayed essentially the
same until 1978, when in ICRP Publication 26 a recommendation was
made to raise the levels of radiation permitted to humans from
man-made sources of radiation (excluding that for medical purposes). For
`internal consistency' of the recommendations there was some
valid argument for scaling the standards for particular organ exposure
in proportion to whole body exposure recommendations -- but scaling
down as well as up would have accomplished this. For example, the
ICRP reasoned that if the whole body could receive 5 rem per year,
the active bone marrow should not be limited to 5 rem per year. This
was used as a reason for increasing the permitted bone marrow dose
from 5 rem to 42 rem with apparently little regard for the increased
damage to bones and blood-producing organs.
ICRP
Publication 26 also reiterates the need to allow human
exposure in order to enjoy the `economic and social benefits' of the
nuclear industries. It is difficult to understand how this conclusion was
reached when so much new research is available documenting human
illness associated with the present permissible exposure
levels.[32]
Perhaps, in view of contemporary scientific concern for lowering
radiation exposures, ICRP Publication 26 recommendations are a
political move to hold the line at present regulatory levels. At any
rate, it appears to be a document with a political rather than a
scientific purpose.
Some
national regulatory agencies, such as the Atomic Energy
Control Board in Canada, promptly implemented ICRP Publication
26 by increasing allowable radium levels in drinking water, thus
reducing the clean-up cost for the uranium mining companies. Since
some members of the national radiation protection community in
Canada and elsewhere hold seats on ICRP, responsibility for what
they recommend nationally cannot credibly be attributed to an
international recommending body.
Failure to Audit Health
ICRP Publication 2 (1959) is one of special interest since it clearly
states that radiation-induced severe genetic defects and cancer deaths
resulting from the recommended standards would be expected to be
rare and hardly distinguishable from `natural' variations due to
non-radiation causes. The document goes on to point out that mild
mutations in offspring and general ill health in those exposed would
be the most frequent health effects of exposure, but these could not be
`detected' except by epidemiological surveys. ICRP Publication 2
made no recommendation that this more subtle widespread degradation of
public health be measured, although they mentioned that it
could be measured.[33]
At no time has there been an effort on
the part of governments to document fully the more subtle health
effects.
Workers,
military service personnel and the general public have
been given the impression that exposure to radiation involves a slight
risk of dying of cancer and that one's chances of escaping this are
better than the chances of escaping an automobile accident. The
probabilities of early occurrence of heart disease, diabetes mellitus,
arthritis, asthma or severe allergies -- all resulting in a prolonged state
of ill health -- are never mentioned. Most people are unaware of the
fact that ionising radiation can cause spontaneous abortions,
stillbirths, infant deaths, asthmas, severe allergies, depressed immune
systems (with greater risk of bacterial and viral infections),
leukaemia, solid tumours, birth defects, or mental and physical
retardation in children. Most of the above-mentioned tragedies affect
the individual or family unit directly and society only indirectly. Dr
R. Mole, a member of ICRP and the British NRPB, stated: `The most
important consideration is the generally accepted value judgment that
early embryonic losses are of little personal or social
concern.'[34]
There are similar value judgments made with respect to other health
effects. The health problems are externalised, i.e. placed beyond the
responsibility of government, and they
are borne by individuals and their families.
The
risk/benefit decision making which arose from balancing `health
effects' against `economic and social benefit' is based on risk and
benefit to society, i.e. governments, rather than cost to the individual
or family unit. Value judgments have been made as to the level of
health effects and deaths `acceptable' to the public. Because of
military control of A-bomb studies and military need for personnel to
handle radioactive materials, many of these value judgments were
cloaked in secrecy for the sake of `national security'. The subject was
made to seem complicated to outsiders; the decisions were reserved
for the experts. The now famous words of President Dwight
D. Eisenhower, `Keep the public
confused'[35]
about nuclear fission so that
the government could gain public acceptance of above-ground weapon
testing in Nevada, have certainly been accomplished. A growing
number of people in the USA and elsewhere have lost all faith in
statements made by government officials, because of the scientific
jargon used to mask the truth.
In
the USA, external radiation exposure records (film badge and
TLD[a]
readings) are carefully kept for workers, but corresponding
health records for workers are not kept and analysed nationally. In
other countries, especially those with socialised medicine, excellent
health records are kept but accurate radiation exposure records are
neglected. Collection and analysis of radiation exposure records
together with experience of ill health, including chronic long-term
(non-fatal) problems, are required in order accurately to assess
radiation-related health problems. Merely recording the first cause of
death for workers is not sufficient.
- TLD -- thermoluminescent dosimeter, used to measure individual radiation doses of workers. It contains radiosensitive chips and must be carefully screened for the kind of radiation it is meant to detect. In a pilot study done in the US some processors of TLDs discovered that some of their chips were completely insensitive to the type of radiation for which they were purchased. See P. Plato and G. Hudson, `Performance Testing of Personnel Dosimetry Services: Alternatives and Recommendations for a Personnel Dosimetry Testing Program', US Nuclear Regulatory Commission (NUREG/CR-1593), 1980, p. 9.
The
public is at an even greater disadvantage than the worker. There
are no cumulative records of radiation exposures for individual
members of the public from nuclear testing, military or commercial
nuclear industries anywhere in the world. Because of this
record-keeping vacuum, it is difficult, if not impossible, to
challenge ICRP predictions.
Inadequate
collection of information on public health by
governments makes it difficult for scientists concerned about rising
radiation exposure levels to document changes in public health. The
problem is not that they are poor scientists, but that they do not have
access to detailed information, since governments have failed to
collect it. The health changes which can be detected, in spite of poor
records, represent only a minute proportion of the undocumented
whole.
One
key to understanding what priority a country places on the
health consequences of national defence and energy choices is the
precision of its measurements of resultant health effects. Measurements
of health effects can be made through controlled
animal experiments or observation of the effects of unplanned human
exposures. These measurements serve as an audit of human health
effects or as an after-the-fact check on the accuracy of predictions. This
technique of controlled observation is normally applied when a
new drug or new medical procedure is introduced into general use. A
prediction must prove its worth in real life.
As
one would expect, predictive dose/response estimates for
radiation exposure and specifically chosen severe health effects have
been prolific in the USA. Not only has the USA maintained a tight
control over and interest in research on the Japanese survivors of
radiation exposure from the nuclear bombing of Hiroshima and
Nagasaki, it also has a system of government-sponsored research
laboratories controlled successively by the Atomic Energy
Commission, the Energy Research and Development Administration
and the Department of Energy. These bodies have been the source of
almost all the original research papers published between 1945 and
1977 on the health effects of ionising radiation. Because
radiation-related health effects are the result of the production, testing
and use of atomic weapons, military goals and military secrecy have
influenced both the selection of research questions and release of
findings in the USA. The nuclear age is predicated on public
acceptance of its consequences, hence `proving' that public
acceptance is `rational' has a very high priority for government and
industry-employed scientists. They have a vested interest in verifying
the status quo.
Prior
to the above-ground nuclear weapon test ban in 1963, the USA
set off at least 183 atmospheric nuclear tests, more than all the other
nations of the world combined. About half these tests were set off near
the Pacific Trust Territory of Micronesia, given into US protection by
the United Nations after the Second World War, and
the other half were set off on the 1,350 square miles at the Nevada
Test Site north of Las Vegas. By 1978 the USA had set off an
additional 400 nuclear bombs below ground in Nevada, some of which
were officially admitted to have `leaked' large amounts of radioactive
chemicals. Some of the tests were of UK weapons since it also uses
the Nevada test site. Underground tests are still taking place in the
USA,[36]
the USSR and French Polynesia. In the Northern Hemisphere,
above-ground tests have also been detonated by the USSR, China and
India and in the Southern Hemisphere by France and South Africa.
The
Nevada nuclear tests have spread radiation poisons throughout
central and eastern United States and Canada, and produced in the
stratosphere a layer of radioactive material which encircles the
globe. They also cause nitric oxides to form in the atmosphere which then
descend on earth as acid rain. Radioactive chemicals can now be
found in the organs, tissues and bones of every individual in the
Northern Hemisphere, and the contamination from past nuclear
explosions will continue to cause environmental and health problems
for hundreds of thousands of years, even if all nuclear activities are
stopped today. Siberian tests affect the north polar region.
Pollution
of the Southern Hemisphere, though less than in the North,
is progressing along the same path. Although the United States and
Great Britain have ceased nuclear tests in the Pacific Ocean, France
has not ceased them, and it appears that South Africa has begun to
test. Brazil, Argentina and other nations are thought to be developing
a nuclear weapon capability.
A
1977 report of the United Nations Scientific Committee on the
Effects of Atomic Radiation stated that twenty atmospheric nuclear
tests -- six in the Northern Hemisphere and fourteen in the Southern
Hemisphere -- plus unnumbered underground tests, took place between
1972 and 1977. As a result of this nuclear testing radiation doses to
the population increased by about 2 percent in the Northern
Hemisphere, and 6 percent in the Southern Hemisphere over the dose
estimated in 1970. The nuclear weapon testing carried out between
1972 and 1977 was insignificant when compared to that between 1945
and 1963.
The total global dose commitment for each individual from all nuclear explosions carried out before 1976 ranges from about 100 mrad (in the gonads) to about 200 mrad (in the bone-lining cells). In the northern temperate zone the values are about 50 percent higher, and in the southern temperate zone about 50 percent lower than these estimates.[37]
This estimate does not include the dose from radioactive carbon
(carbon 14) which, because of its 5730-year half-life, persists in the
human food chain and has not yet taken its total human toll. For
comparison purposes, 100 mrad is about equal to the amount of
radiation a person receives from naturally occurring radiation in one
year of chronological ageing. The dose commitment from nuclear
weapon testing is spread over a fifty-year period, with most of the dose
being delivered in the first year.
There
has been no lack of victims of radiation pollution in the West
to study both for refinement of predictions of biological harm and
checks of adequacy of predictions relative to the real-life
situation. Checking adequacy of predictions means including
all hidden costs which must eventually be paid, including damage to
agriculture and the biosphere. Government oversight should also include full
disclosure of findings to the public as a test of the acceptability of
such costs and as an evaluation of the judgments made for society by
the nuclear experts.
Can Health be Measured?
The obvious answer is that we can, of course, find a way to measure
gains and losses in health; only the will to do so is lacking. In order to
measure subtle changes in health a good reporting and recording
system is needed, together with protection of privacy for the individual
and ongoing biostatistical analysis of the accumulated data. Whole
bodies of statistical theory, such as sequential analysis, used for
product quality control, and system analysis, used to predict the
outcome of a complicated interaction between interdependent
variables, need to be used in the public health sector. This could
provide a public health technology capable of managing military and
industrial technology, able to act as a reality check on predictions and
to give an early warning of dangers arising from within the big-system
and threatening survival of the nation or, indeed, the human
race. Biostatistical detection of problems needs to be followed by
pathological, cytological and other confirmatory studies. No such
serious systematic commitment to public health is evident relative to
this nuclear issue anywhere in the world. Governments seem unaware
that economic and military policies can
be destructive of human health within the nation.
The
radiation issue is further confused by statisticians and public
health specialists who claim that there are some inherent and
insurmountable problems which make it impossible to monitor the
public health effects of
pollution.[38]
These professionals seem to limit
themselves, consciously or unconsciously, to current inadequate data
collection systems and mathematical tools. This is like deciding that
it is impossible to travel to the moon on the basis that the only
transportation possible is a commercial airliner. It will very probably
require grass-roots scientific initiatives to cause governments to begin
to act as strongly in protection of public health as they act to promote
their own economic and military strategies.
Many
people have become aware that national security strategies,
especially nuclear weapon stockpiling, are increasing individual
insecurity. Capital-intensive national economic strategies, designed to
balance import/export dollar flow, can cause havoc with the
individual citizen who is having to cope with the side effects of
inflation and unemployment. Government neglect of health monitoring
relative to economic and military strategies is, however, not yet
perceived by the public as a serious problem.
It
should be obvious that pollution of the environment with fission
products will cause a wide variety of physiological changes in people
exposed to them. There is little disagreement among scientists with
regard to this conclusion.
There
is also little controversy about the tragedy caused by
uncontrolled fission -- whether deliberately or accidentally unleashed,
whether from a nuclear reactor accident or an exploding warhead.
The
question which causes controversy is: which health effects
should be recognised as important for fiscal planning? `Important' may
relate to public acceptance of the problem, or to the money which
must be paid out for damage compensation, or the productive years
lost through premature disability or death of workers. Once the
significant health effects are identified, then quantification of these
effects becomes the primary societal goal. This gives rise to scientific
controversies. Present scientific controversy on low level radiation has
to do with estimating the number of radiation-induced `excess cancer
deaths' that are related to a given dose of ionising radiation. Fiscal
concern has centered on radiation-induced excess cancers, and
scientific concern on predicting this outcome.
These
excess cancer numbers are important to planners who wish
to show that their development schemes are less harmful than an
alternative scheme. They are important to government officials who
have to decide whether or not to assume the financial burden of
ordering evacuation of a danger zone in a reactor accident like that at
Three Mile Island. They are important to insurance companies, since
they allow calculation of theoretical liability due to an accident. They
are important to legislators who need to balance risks (deaths) against
some military or economic benefit. They are important to strategic
planners who calculate `collateral damage', i.e. the number of human
deaths, after an atomic attack.
These
numbers of specifically selected health effects, `radiation-induced
excess cancer fatalities', predicted on the basis of the `average man's'
reaction to a given average dose of ionising radiation, are of little
meaningful use to individuals. Firstly, no one is really
an `average man'. Also, populations may vary in the proportion of
people with above-average susceptibility to radiation
damage. Secondly, a `radiation-induced excess cancer fatality' is
one of the least likely of the health problems to occur with exposure
to low level radiation. More likely scenarios are radiation
acceleration of a cancer caused by some other factor, such as cigarette
smoking[b],
earlier clinical expression of cancer, benign tumours, or
related non-malignant health problems. Thirdly, even if the individual has a
cancer it is almost impossible to present evidence to prove that his or
her cancer is the excess one which would not have occurred without the
radiation exposure. Therefore compensation for damage is almost impossible
to obtain. Only one veteran from the USA exposed to radiation in its nuclear
bomb programme has ever received compensation: Orvile Kelly. About six
months before he died the Veterans Administration admitted that his illness
could be attributed to radiation exposure. About 1,000 veteran claims have
been refused.[39]
- Many researchers believe that the primary carcinogen in cigarettes is polonium 210, a radioactive daughter product of radon gas which is released from uranium mining and i mill tailings.
The
usual `rational' approach to risk versus benefit planning by
governments is irrational from the point of view of the individual. It
undermines the individual's ability to control and understand his or her
environment and to hold government accountable to its electorate.
The
human body is delicately fashioned and the unique gifts of
each person are meant to enrich the human family. Crude
quantification of random damage to people which is used to justify
political or military gains of the nation may be labelled sophisticated
barbarianism. It is the decadent thinking of those who have accepted
the rule of force and who envision a future earth ruled by a powerful
country (the USA or the USSR) with a monopoly of weapons of mass
destruction, able to terrorise all other nations into co-operating with
some form of global economy and resource-sharing of their choosing.
The Health Physicist
A word needs to be said about health physics, a relatively new
academic specialty which has emerged since the dropping of the
atomic bomb. Systematic study of radiation health questions began at
the University of Chicago when the first nuclear reactor began
operating on 2 December 1942. Primarily under the leadership of
physicists E. O. Wollan, H. M. Parker, C. C. Gamertsfelder,
K. Z. Morgan, J. C. Hart, R. R. Coveyou, O. G. Landsverk and L. A. Pardue,
it grew to become a recognised graduate-level
discipline.[40]
While
this was a much-needed specialty, its bias toward the
so-called `hard sciences' -- physics, chemistry and engineering -- and
neglect of the `soft sciences' -- biology, physiology and psychology --
has tended to create radiation safety officers rather than health
professionals.
In
a message from the President of the Health Physics Society
published in the July 1971 issue of the Health Physics
Journal,[41]
Dade W. Moeller stated:
I think it is interesting to note the results of a tabulation of the records of the 2,862 health physicists who joined the Society from 1960 through 1969. The data showed that although half of the new members with college degrees had attended graduate school for a year or more, 21.6 per cent of the new members did not have a college degree. [Emphasis added.]
Membership of the Health Physics Society is broader than, but
includes, licensed health physicists who have passed qualifying
examinations. These latter are generally required to have a college
degree with a major in physics, chemistry or engineering, and one
year of graduate training in radiation measurement and safety
practices.
Dade
W. Moeller goes on to describe the members who had a
college degree:
by far the greatest percentage (24.0 per cent) received their bachelor's degrees in physics and/or mathematics. Next was chemistry (15.8 per cent) and then engineering (13.6 per cent).
Even members of the Health Physics Society have complained about
the pro-nuclear bias of its
publication[42]
but seldom has this been
expressed as clearly as in this address by Dade Moeller. After
reporting a need for 2,000 to 3,000 more health physicists by the year
2000 just to support the operation of nuclear power stations, he urged
members to be active: `To paraphrase an old adage, "let's all put our
mouth where our money is".'
Unfortunately,
the Health Physics Society probably will not be in
the vanguard speaking on behalf of workers and members of the
public whose health is at risk from nuclear industries. The obvious and
outstanding exception to this statement is Dr Karl Z. Morgan who has
remained an open, honest and independent student of life. Dr Morgan
has spoken out courageously on behalf of lowering worker and public
exposures to radiation and avoiding all unnecessary exposures. In so
doing he has alienated many of his peers and jeopardised his own
research and teaching position. Karl Morgan was a friend of Hermann
Müller and he remembers the geneticist's warning about undermining
the health of a nation and its
children.[43]
The
United States, a leading nuclear nation, has failed to provide
any reliable human health study either to confirm or to deny its
prediction of the human health effects of exposure to chronic low
level radiation, or even to provide a systematic health follow-up of
the significant groups exposed to radiation so that there will in time
be such a reliable study. The predictions of health effects are based
primarily on the effects reported at Hiroshima and Nagasaki and the
applicability of these estimates to chronic low dose exposure of a
normal population has always been
doubtful.[21]
The
US government has also failed to supply the worker or the
public with trained health professionals whose jobs are independent of
the nuclear industry and whose training and background would enable
them to alert people to a slowly deteriorating health situation. Adequate
record-keeping and reporting would force public awareness
of the problems, and probably the facing of ultimate questions such
as: for what perceived benefit can society sacrifice the health of
future generations?
The
health physicist, while serving a necessary safety function
within nuclear installations, does not fulfil the role of a health
advocate in this situation. His or her job is to enforce regulations, not
to question them and to support the nuclear plant management even if
it is clear that the management is
wrong.[44]
This is not so much the result of
malice as a normal outcome of believing `permissible' is the same as
`safe', and trust that present regulations are `very safe'. It thus
becomes acceptable to handle radioactive material and to cheat a
little on over-exposures.
The
first key to understanding governments' commitment to
ensuring the survival of individual citizens is its adoption of a
verification process for testing its prediction of severe health effects
resulting from its economic and military strategies. In the United
States, this leads to a preliminary judgment that individuals have
been considered expendable. Health damage from radiation
associated with military or economic ventures has not been easily
traceable to the cause or immediately apparent to the public. No
efforts deliberately to trace and make public all the health effects
have been made. In fact, when any research has begun to show such
effects, the researcher has been `discredited' and his or her funding
discontinued.
On
the basis of the US government's neglect of follow-up and
record keeping on radiation-exposed people, and its lack of concern
for mild genetic effects, the unrest of the US public with respect to
further development of nuclear technology is highly rational. Continuance
of present government neglect and unconcern is at best
irrational and at worst genocidal. We may observe the same syndrome
of irrational behaviour in other nuclear nations which are experiencing
public unrest.
Although
the problems inherent in the production of nuclear
weapons and nuclear power reach a climax of scale in the United States,
they are experienced in all countries with nuclear technology. Where
one country may keep excellent public health records, it has
poor records of individual radiation exposures. Where another keeps
detailed radiation exposure histories, it has no detailed medical
history. As long as part of the information is missing, the worker and
general public are forced to rely on predictions made by `recognised
experts' which are not verified by factual studies. This is really a
forecast with no audit allowed. The promotion of nuclear technology
in developing nations as the industry loses support in the developed
world is even more disturbing.
Before
moving on, some of the concepts of radiation protection
important for nuclear workers, the general public and medical
personnel need to be emphasised. First, an assurance of `no immediate
danger' with respect to exposure to ionising radiation is empty when it
masks long-term effects resulting from incorporation of radiochemicals
in sensitive tissues and/or the results of biological magnification of
cell damage or radiation-induced genetic mistakes. Secondly,
independent testing of urine, faeces, exhalation, tissues removed in
surgery, baby teeth and hair for radioactivity, must become routine
laboratory tests for medical diagnostic purposes as we try to cope with
the fission product pollution already in the biosphere. Thirdly, when
assessing the impact of any leak, abnormal release, normal effluence
or waste which is radioactive, it is essential to know the
radiochemicals involved: their physical and biological properties, the
potential pathways to human beings and the length of time they
remain toxic. Fourthly, the health effects of radiation differ with the
age of the person exposed, his or her physical status and prior
experience.
The
second key to governmental priorities in decision-making is
found in the historical context of the nuclear development. This is
examined later. First we must try to understand the practices of
nuclear technology in the military and civil sectors.
References:
-
`Fission Products' in The Effects of Nuclear Weapons
(3rd edn), US Department of Defence and US Department of Energy,
1977, Section 1.60 to 1.66.
-
Karl Z. Morgan, `Suggested Reduction of Permissible
Exposure to Plutonium and Other Transuranium Elements', American
Industrial Hygiene Association Journal, August 1975, pp. 567-75.
-
Karl Z. Morgan, `Hazards of Low-Level Radiation',
Yearbook of Science and the Future, Supplement of the
Encyclopedia Britannica, 1980.
-
Karl Z. Morgan, `Reducing Medical Exposure to Ionizing
Radiation', American Industrial Hygiene Association Journal,
May 1975, pp. 361-2.
-
H. Müller, `Radiation and Heredity', American
Journal of Public Health, vol 54, no. 1, 1964, pp. 42-50.
-
R. Bertell, `X-ray Exposure and Premature Aging', Journal
of Surgical Oncology, 9, 1977, pp. 379-91.
-
N. Kochupillai, I. C. Verma, et al., 'Down's Syndrome and
Related Abnormalities in the Area of High Background Radiation in
Coastal Kerala', Nature, 262: 60, 1976.
-
P. M. E. Sheehan and I. B. Hillary, `An Unusual Cluster of
Down's Syndrome, Born to Past Students of an Irish Boarding School',
British Medical Journal, vol 287, 12 November 1983. Also
Letters and Author's reply, British Medical Journal, vol
288, 14 January 1984.
-
L. S. Penrose and G. F. Smith, Down's Anomaly,
Churchill, London, 1966.
-
Carl J. Johnson, `Cancer Incidence in an Area of Radioactive
Fallout Downwind from the Nevada Test Site', Journal of the
American Medical Association, vol 251, no. 2, 13 January 1984.
-
H. B. Jones, `Estimation of Effect of Radiation Upon Human
Health and Life Span', Proceedings of the Health Physics Society,
June 1956.
-
L. H. Gray, `Biological Damage by Different Types of Ionizing
Radiation' in Biological Hazards of Atomic Energy, Clarendon
Press, Oxford, 1950.
-
Karl Z. Morgan, `Risk of Cancer from Low Level Exposure to
Ionizing Radiation', American Association for the Advancement of
Science, Washington, DC, 17 February 1978.
-
R. M. Sievert, `Tolerance Levels and Swedish Radiation-protection
Work', Proceedings of the Health Physics Society, June 1956, p. 181.
-
M. Eisenbud, `Radioactivity in the Environment -- Radioactive
Concentration in the Fetal Thyroid', Pediatrics (Supplement),
41, Part II, 1968, p. 174.
-
ICRP Publication 2, Pergamon Press, Oxford 1959.
-
H. Muller, `Radiation and Heredity', American Journal of
Public Health, vol 54, no. 1, 1964, pp. 42-50.
-
`Biological Effects of Ionizing Radiation', BEIR III, US National
Academy of Science, National Academy Press, 1980, pp. 74-5.
-
R. Bertell, `Ionizing Radiation Exposure and Human Species
Survival', Canadian Environmental Health Review, vol 25,
no. 2. June 1981
-
`Basic Radiation Protection Criteria', US National Council on
Radiation Protection Report no. 39, pp. 58-60.
-
A. M. Stewart, `Delayed Effects of A-Bomb Radiation: A Review
of Recent Mortality Rates and Risk Estimates for Five-year Survivors',
Journal of Epidemiology and Community Health, vol 36, no. 2,
June 1982, pp. 80-6.
-
R. Bertell, Letter to the Interagency Task Force on Low-Level
Ionizing Radiation (director F. Peter Libassi); published in
Public Comments on the Work Group Reports, US Department of
Health, Education and Welfare, June 1979.
-
S. Macht and P. Lawrence, `National Survey of Congenital
Malformations Resulting from Exposure to Roentgen Radiation',
American Journal of Roentgenology, 76, 1955, pp. 442-66.
-
`Biological Effects of Ionizing Radiation'. BEIR 111, US
National Academy of Science, National Academy Press, 1980, pp. 74-5.
-
ICRP Publication 9, Pergamon Press, Oxford. 1965.
-
Recommended by pioneer researchers A. Mutscheller and R. M. Sievert
in 1925. Recommended for international use by the forerunner of the
International Commission on Radiological Protection (ICRP) in
1934. Used in most countries until 1950.
-
Recommended by the US National Commission on Radiological
Protection (NCRP), 17 March 1934.
-
Recommended hy the US NCRP, 7 March 1949 and hy ICRP, July
1950, for total body exposure.
-
Recommended by ICRP, April 1956 and US NCRP, 8 January 1957, for
total body exposure. This allows for 5 rem per year combined dose from
sources external to the body, ingested or inhaled sources. This
standard is used in most countries of the world today.
-
ICRP 26, Pergamon Press, Oxford. 1978.
-
See op cit., note 12, pp. 96 and 123. British, Canadian
and American nuclear physicists met in Chalk River, Canada in September
1949 and at Buckland House, UK in August 1950 to agree on radiation-dose
levels for workers. Their recommendations were accepted by ICRP.
-
Karl Z. Morgan, `Hazards of Low-Level Radiation'. Yearbook
of Science and the Future, Supplement of the Encyclopedia
Britannica. 1980.
-
ICRP Publication 2, Pergamon Press, Oxford, 1959. See also
Statistics Needed for Determining the Effects of Environment on
Health, US DHEW Publication no. (HRA) 77-1459 (1977).
-
R. Mole, `Radiation Effects on Pre-natal Development and their
Radiological Significance', British Journal of Radiology,
52:614, 1979, pp. 89-101.
-
`Ike Sought Confusion Over Nuclear Testing', Associated Press
Report, 20 April 1979.
-
For information on past and present nuclear tests in Nevada
contact: Citizen's Call, 1321 East 400 South. Salt Lake City, Utah
84102, USA. Official reports on nuclear tests in Nevada are available
from the US Department of Energy which operates the test
site: Announced United States Nuclear Tests, July 1945 to
December 1983. NVO-209 (REV.4), January 1984.
-
`Sources and Effects of Ionizing Radiation', UN Scientific
Committee on the Effects of Atomic Radiation, Report to the General
Assembly, nos. 90-91, 1977.
-
C. E. Land, `The Hazards of Fallout or of Epidemiologic Research',
Editorial in New England Journal of Medicine, 300 (8): 431-2, 1979.
-
For ongoing information on atomic veterans contact: International
Association of Atomic Veterans, 236 Massachusetts Avenue. N.E., Suite 306,
Washington, DC 20002, USA (tel: 202-543-7711).
-
Karl Z. Morgan and J. E. Turner (eds). Principles of Radiation
Protection, A Textbook of Health Physics, John Wiley, New York,
1967. See Preface.
-
Dade Moeller, `The President's Message', Health Physics
Journal, vol 21, 1971, p. 1.
-
See dialogue in Letters, The Health Physics Society
Newsletter, vol XII, May and August 1984.
-
Karl Z. Morgan, `Hazards of Low-Level Radiation'. Yearbook
of Science and the Future, Supplement of the Encyclopedia
Britannica, 1980.
-
Personal correspondence to the author from Karl Z. Morgan, first
Chairperson of the Committee on Internal Exposures, ICRP and director
of the health physics programme at Oak Ridge National Laboratory and
Neely Professor, School of Nuclear Engineering, Georgia Institute of
Technology.
