Measuring Radiation
One way to approach the measurement of radiation is to count the
number of nuclear transformations or explosions which occur in a
given unit of radioactive substance per second. This measure is
usually standardised to radium, the first radioactive substance to be
discovered and widely used. One gram of radium undergoes 3.7 x 10^10
nuclear transformations or disintegrations per second. The activity of 1
gram of radium is called 1 curie (Ci), named for Madame Marie
Curie, a Polish-born French chemist (1867-1934). Marie Curie
discovered the radioactivity of thorium, polonium and radium by
isolating radium from pitchblende. She and her daughter Irene were
among the earliest known radiation victims, both dying of aplastic
anaemia.
In
recent radiation protection guides, the curie is being replaced by
the becquerel, which indicates one atomic event per second. One
gram of radium would equal 1 curie of radium or 3.7 x 10^10 becquerels
of radium.
The
energy released in nuclear disintegrations has the ability to
do work, i.e. to move matter. In physics, the erg is a very small unit of
work done. Lifting 1 gram of radium 1 centimetre requires 980 ergs of
work. Any material exposed to the force from nuclear disintegrations
at a rate of 100 ergs/gm is said to absorb one rad, i.e. radiation
absorbed dose. There is no direct conversion from curies,
which is related to the number of atomic events, to the rad dose, which
is energy absorbed in tissue. The curie gives one an estimate of the
number of microscopic transformations or explosions per second and
the rad is an estimate of the energy release, absorbed by the
surrounding tissue. On the macro-level, the word `explosion' tells us
only of an event in time. A dynamite explosion or hydrogen bomb
explosion adds information about the energy released.
Sometimes
radioactivity is measured in counts per minute on a
Geiger counter. A nuclear transformation within an energy range
measured by the instrument and close enough to the instrument
causes a noise or `count'. Most Geiger counters cannot detect alpha
particle emitters like plutonium.
The
radioactivity of elements which experience nuclear
disintegrations is measured relative to radium. For example, it would
take more than 1 million grams of uranium to be equivalent in
radioactivity, i.e. to have the same number of nuclear events per
second as 1 gram of radium has per second. Both 1 million grams of
uranium and 1 gram of radium would be measured as 1 Ci. It has been
the custom in the past to limit human exposure to uranium more for its
toxic chemical properties (it is a heavy metal) than for its
radioactivity. This practice may have underestimated damage caused
by the biological storing of uranium in the liver.
When
uranium decays, it passes through about 12 radioactive forms,
called daughter products, before reaching a stable chemical form of
lead. One of the radioactive daughter products of uranium is
radium. Uranium released into drinking water or incorporated into food
and human tissue today will eventually plague the world as radium and its
other disintegration products: radon gas and the radioactive forms of
polonium, lead and bismuth. The environmental and biochemical
forces which may tend to reconcentrate these toxic materials in living
cells are not well known. Although uranium occurs naturally, it has
become much more available for entering into water, food, living cells
and tissue since the mining boom which began shortly after the
Second World War.
The
activity which takes place in the nucleus of the uranium or
radium atom is a `haphazard' event obeying the laws of random
probabilities. An atom is characterised by its atomic number, that
is, the positively charged particles in its nucleus, and by its atomic
mass, expressed in atomic mass units (similar to the concept of
weight), which includes both the number of protons (the atomic
number) and the number of neutrons in the nucleus. Carbon, the most
frequently occurring chemical in living material, is taken as having
exactly 12 atomic mass units and other atoms are measured in
relation to this. Carbon 14, which is radioactive, has two extra
neutrons in its nucleus.
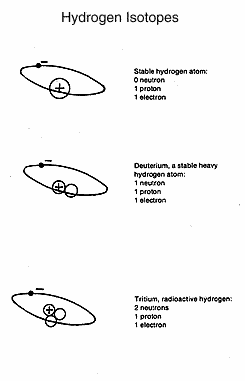 Hydrogen,
another example, has an atomic number of 1 and an
atomic mass of 1. Isotopes of hydrogen have the same atomic number
(that is, the same number of positively charged particles in the
nucleus and electrons in orbit around the nucleus) but a higher atomic
mass. Deuterium or hydrogen 2, an isotope of hydrogen, has an atomic
number of 1 and an atomic mass of 2. It is not radioactive. The
increased atomic mass is due to an added neutron in the
nucleus. Deuterium is in the `heavy water' used in the Canadian CANDU
nuclear reactor. Hydrogen 3, called tritium, is radioactive, with two
neutrons and a proton in the nucleus. It is produced in a nuclear
reaction.
Hydrogen,
another example, has an atomic number of 1 and an
atomic mass of 1. Isotopes of hydrogen have the same atomic number
(that is, the same number of positively charged particles in the
nucleus and electrons in orbit around the nucleus) but a higher atomic
mass. Deuterium or hydrogen 2, an isotope of hydrogen, has an atomic
number of 1 and an atomic mass of 2. It is not radioactive. The
increased atomic mass is due to an added neutron in the
nucleus. Deuterium is in the `heavy water' used in the Canadian CANDU
nuclear reactor. Hydrogen 3, called tritium, is radioactive, with two
neutrons and a proton in the nucleus. It is produced in a nuclear
reaction.
When
radium 226 decays, it loses a positively charged alpha
particle from its nucleus. An alpha particle has two protons (positive
electrical charges) and a mass of 4 atomic units. This means a
reduction in both radium's atomic number and atomic mass. Loss of
the alpha particle changes radium 226 (transmutes it) into another
element, radon 222. While radium 226 is a radioactive solid under
normal conditions, radon 222 is a radioactive gas. Loss of one or more
protons changes the chemical element into a different
chemical. Absorption or loss of a neutron gives an isotope of the
same chemical since chemical properties are determined by the number
of protons and electrons in an atom.
The
time required for half of any amount of radium 226 to transmute
to radon 222 by these small explosions which emit alpha particles
is 1,622 years. This is called the physical half-life of radium. Half
of the radium literally disappears in that length of time, but radon
gas is produced to replace it. Radon gas is radioactive and more
mobile in air and water (it dissolves) than the solid radium. The
half-life of radon is 3.82 days, after which half the gas will have
disintegrated, again releasing alpha particles and transmuting into
radioactive polonium 218, which is a solid. With a wind of 10 mph (or
kph), the radon gas could travel 1,000 miles (or kilometres) from the
point of origin before half of it would have decayed into its solid
daughter products and been deposited on soil, leafy vegetables,
tobacco, groundwater, human skin, lung tissue, etc. If the
material receiving the radioactive daughter product is living,
then it can carry the particles into its cells. Such contamination
cannot be washed off.
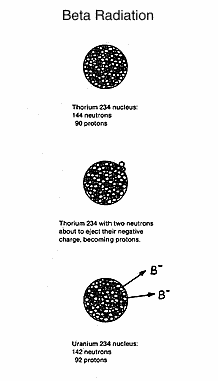 When
a negatively charged beta particle is released, there is a
transmutation in which a neutron in the nucleus of the atom splits into
a proton and an electron, the proton remaining in the nucleus and the
electron given off as a fast-moving microscopic bullet. Beta particles
are extremely small. The mass of an alpha particle is about 7,400
times that of a beta particle. Thorium 234 decays to uranium 234 (with
a short-lived radioactive intermediary) by losing beta particles. Uranium
and thorium are different elements, but have the same mass
(atomic weight) since a neutron and proton have about the same
mass. The thorium neutron becomes the uranium proton. The half-life
of thorium 234 is 24.1 days, while the half-life of uranium 234 is 2.50
x 10^5, or 250,000 years. As was pointed out earlier, uranium nuclear
events are not as frequent as those in radium, although they are
destructive when they occur.
When
a negatively charged beta particle is released, there is a
transmutation in which a neutron in the nucleus of the atom splits into
a proton and an electron, the proton remaining in the nucleus and the
electron given off as a fast-moving microscopic bullet. Beta particles
are extremely small. The mass of an alpha particle is about 7,400
times that of a beta particle. Thorium 234 decays to uranium 234 (with
a short-lived radioactive intermediary) by losing beta particles. Uranium
and thorium are different elements, but have the same mass
(atomic weight) since a neutron and proton have about the same
mass. The thorium neutron becomes the uranium proton. The half-life
of thorium 234 is 24.1 days, while the half-life of uranium 234 is 2.50
x 10^5, or 250,000 years. As was pointed out earlier, uranium nuclear
events are not as frequent as those in radium, although they are
destructive when they occur.
Given
12 grams of thorium 234, we would have 6 grams after 24.1
days, 3 grams after 48.2 days, 1.5 grams after 72.3 days, 0.75 grams
after 96.4 days, etc. At the same time, the stock of uranium 234 would
be increasing as the thorium decays into the new radioactive
chemical.
There
is no simple physical or chemical process such as
temperature change or chemical bonding which can prevent these
radioactive elements from decaying. Their nucleus is unstable and
because all elements seek a stable low-energy state, they must at
some time release particles in an effort to reach a resting state. The
decay takes place in the nucleus of the atom regardless of whether the
atom exists singly or is part of a molecule; is in the solid, liquid or
gaseous state; is within the body or outside, and so on. The decay
product after a radioactive disintegration may itself be radioactive, so
disintegration does not put an end to the biological problems
generated by these small explosions. This decay process must be
taken into account when estimating the biological effects of internal
exposure to radioactive material. Inhaled radon gas quickly becomes
radioactive lead, bismuth or polonium in the bloodstream.
One
should not confuse physical half-life with biological half-life,
i.e. the time required to eliminate half of the material from the body
through exhalation, urine or faeces. Cesium 137 and strontium 90 both
have physical half-lives of almost thirty years, but cesium 137 is
normally excreted from the body within two years while strontium 90
can be incorporated in bone for a lifetime.
One
more measure needs to be introduced before radiation protection
guides can be understood. Since the various kinds of radiation
exposures need to be evaluated for biological impact and not just for
the amount of energy absorbed by the tissue, the term rem, roentgen
equivalent man (or woman), was introduced. The rem dose is
the rad dose times a quality factor Q. For external radiation Q is
usually taken as 1, and rads and rems are used interchangeably. However,
to reflect the greater biological damage done by alpha
particles when inside the body, the rad dose may be multiplied by 20
to give the rem dose. This is another way of saying that the alpha
particle does damage of an order of magnitude (20 times) greater
when lodged within a tissue, bone or organ. For example, alpha
particles giving a 2 rem (or rad) dose to skin would give a 40 rem
dose to sensitive lung tissue when inhaled.
Theoretically,
the rem dose measures equivalent biological effect,
so that damage from X-rays, for example, would be the same as damage
from alpha particles, when the dose in rem was the same. Unfortunately,
living systems are too complex for such an approach to
provide anything more than a good guess.
Sometimes
references are made to a `fifty-year effective dose
equivalent'. This is the full dose that would be received from an
internal radionuclide if the dose were given at one time instead of
being spread over two to fifty years.